As part of the US Food & Drug Administration’s (FDA) continued effort to combat economically motivated adulteration, it conducted an assignment from 2022 to 2024 to collect and test imported frozen raw and cooked seafood for short weighting (i.e., product is offered for sale containing less than the declared net weight on the package). Short weighting in frozen seafood can occur if the net weight of the product includes unacceptable weight of the water glaze, or ice.
It is common industry practice to add water glaze to frozen seafood products to help protect them from freezer burn while in extended storage. The longer the product may be frozen, the thicker the typical glaze may be. However, overstating the net weight of frozen seafood by including the weight of glazing (ice) is not permitted (see FDA’s Guidance for Industry: 1991 Letter to Seafood Manufacturers Regarding the Fraudulent Practice of Including Glaze (ice) as Part of the Weight of Frozen Seafood). Overstating the net quantity of contents (such as including the weight of ice glaze) is a type of food fraud, where consumers are cheated by providing misleading information about the value of the packaged seafood based on weight.
The purpose of the 2022-2024 assignment was to identify imported packaged frozen seafood products that were violative for short weighting and prevent any adulterated or misbranded products from being distributed in US commerce channels. This assignment identified that 36% of the samples (10 out of 28) were violative for short weighting.
Methodology
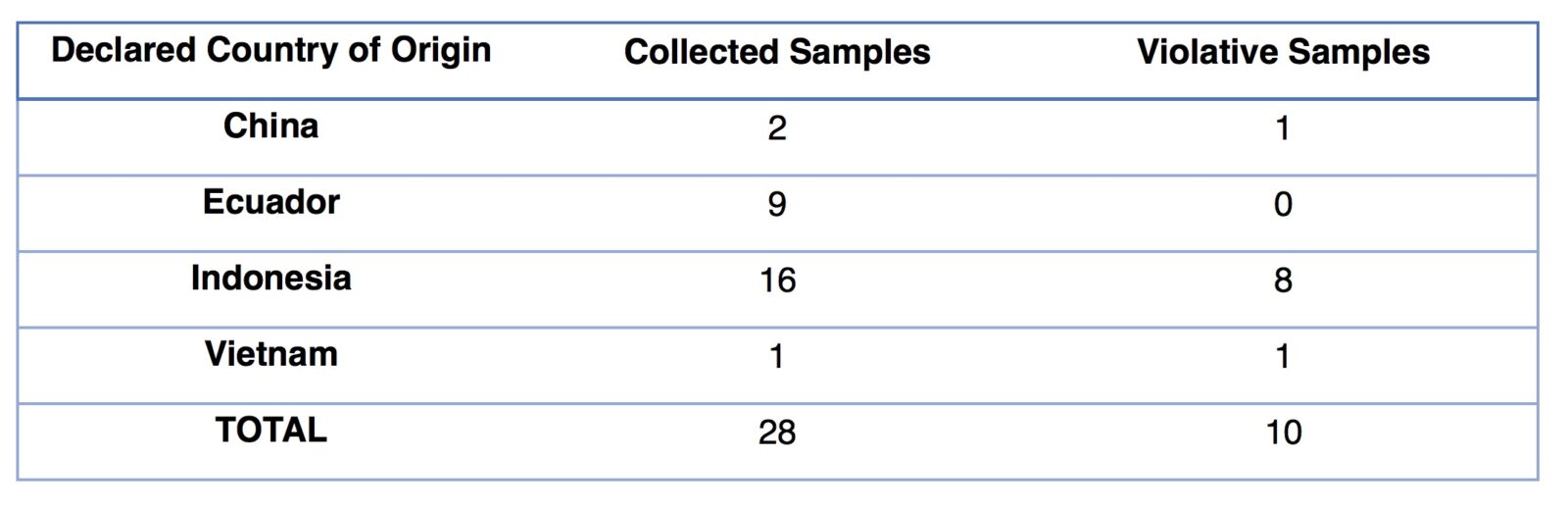
The agency collected and tested 28 samples of retail packaged frozen seafood products to determine whether they met the net weight declared on the product label. The samples of 25 shrimp, two squid and one tilapia product included both targeted samples based on complaints received by FDA and general surveillance samples. The seafood was imported from 12 unique firms in four countries (China, Ecuador, Indonesia and Vietnam).
Each sample consisted of 48 units of packaged frozen seafood product from the same production lot. The retail packs ranged in net weight from 2 to 7 lbs. FDA field personnel collected the samples in import status, which refers to products at ports of entry or other locations where they are held prior to being released into the US marketplace.
The samples were evaluated by FDA laboratories for net weight using the Association of Official Analytical Chemists (AOAC) method 963.18 – “Net contents of Frozen Seafoods – Drained Weight Procedure.” When a sample showed an average short weight of one percent or more, the results were referred to FDA compliance for potential regulatory actions.
Findings
Of the 28 imported samples collected and tested, the FDA found 10 (36%) to be violative, with percent short weighting ranging from 2.3% to 9.9%. The products were imported from five unique firms in three countries (China, Indonesia and Vietnam) and included eight shrimp and two squid samples.
Follow-Up Actions
When the FDA found a sample to be violative, it took follow-up regulatory action to prevent further distribution of adulterated or mislabeled product into US commerce channels, as well as future shipments until corrections were made by the firm. All 10 violative samples resulted in refusal of entry for that shipment into the United States In addition, the FDA placed the associated five companies and their violative products on Import Alert 99-47.
Products listed on the alert are subject to detention without physical examination. For such products, the exporting company may provide evidence to the FDA to overcome the appearance of a violation, such as the test results of a third-party laboratory, verifying the manufacturer did not include the glaze as part of the declared net weight on the package. If FDA concludes that there remains an appearance of a violation, it refuses the product.
The results of this assignment show frozen seafood products continue to be a commodity susceptible to economically motivated adulteration. This reaffirm the need to continue to test frozen fishery products for economically motivated adulteration to ensure consumers are not deceived.
Violative samples are subject to regulatory actions, consistent with the FDA’s mission to ensure that food is safe, wholesome and properly labeled. When appropriate, the agency may consider pursuing criminal investigations. The FDA also collaborates with international counterparts to detect and combat economically motivated adulteration related to imported products, including imported glazed fishery products.